Why is corn syrup used in ice cream?
18 MINUTE READ
If you find the content on this blog useful and would like to support my work, please consider doing so on Patreon.
Sweeteners commonly used in ice cream formulations include cane and beet sucrose (‘table sugar’), maltodextrin, corn syrup, high maltose syrup, dextrose, fructose or high fructose corn syrup, maple syrup or maple sugar, invert sugar, honey, brown sugar, and lactose. Goff & Hartel (2013) note that the most common choice of sweetener in ice cream is a combination of sucrose (10-12%) and corn syrup (3-5%).
You might also like to read:
Cuisinart ICE-100 Ice Cream Maker - A Comprehensive Review
Why are stabilizers used in ice cream?
Sugar in ice cream
How to calculate an ice cream mix
Vanilla Ice Cream - Recipe
1. What is corn syrup?
Corn syrup, commonly referred to as 'glucose syrup' or 'corn starch hydrolysate syrup' (CSS), is a nutritive (or caloric) sweetener produced by the partial hydrolysis of starch. In the United States, corn syrups are made from corn (maize) starch. In Europe and Australia, corn syrups may be made from potato or wheat starch, in addition to corn starch, whilst in Asia, cassava (also known as tapioca) and rice starch are used.
The hydrolysis process is essentially one of breaking down starch into the simple sugar glucose (also known commonly as dextrose), by acid or enzymes, or by a combination of acid and enzyme treatments. This results in hydrolysed syrups consisting mainly of dextrose, maltose, maltotriose, and higher sugars.
1.1. What is Dextrose Equivalence (DE)?
Corn syrups are classified by their dextrose equivalence (DE), which indicates their degree of hydrolysis from starch to dextrose. The higher the DE, the sweeter the corn syrup will be until complete conversion to dextrose, which has a DE of 100, is achieved.
2. Maltodextrins
Corn syrups are defined as having a DE of 20 or greater. Below DE 20, the products are referred to as maltodextrins. Maltodextrins are available as white powders in varying DE values, typically 1, 5, 10, 15, and 18 (Hull, 2010), have a bland taste with little sweetness, and high viscosity. They are used in ice cream to provide smooth and creamy texture, provide body and mouthfeel, contribute solids, produce a firmer ice cream that melts slowly during consumption, and extend shelf-life during storage.
2.1. A fat replacer
Because maltodextrins have a texture similar to fat, they can be used to replace 30-40% of the milk fat in an ice cream mix (Goff & Hartel, 2013).
3. Corn Syrups
Corn syrups are available in a DE of between 20 and 95, generally in a liquid form, although powdered forms are also available. As the DE of a corn syrup increases, so too does its sweetness, flavour enhancement, and effect on freezing point depression, but its viscosity, ability to enhance smoothness and creaminess, contribution of body, mouthfeel, and solids, air bubble stabilisation, and ability to increase the shelf-life of ice cream during storage decrease. Ice cream manufacturers usually use liquid or dry corn syrup products with a 28-42 DE (Goff & Hartel (2013)Below is a table showing the characteristics of sweeteners in ice cream, from Goff & Hartel (2013).
4. Maltose and high Maltose syrup
These syrups are characterised as having less than 10% dextrose and a maltose content that can vary from 28% to 70% or higher (Hull, 2010). Maltose syrups with a maltose content of 28-49% are usually referred to as a 'maltose syrup’, whilst syrups with a higher maltose content are referred to as either ‘high maltose syrups’ or ‘very high maltose syrups’.
5. Dextrose
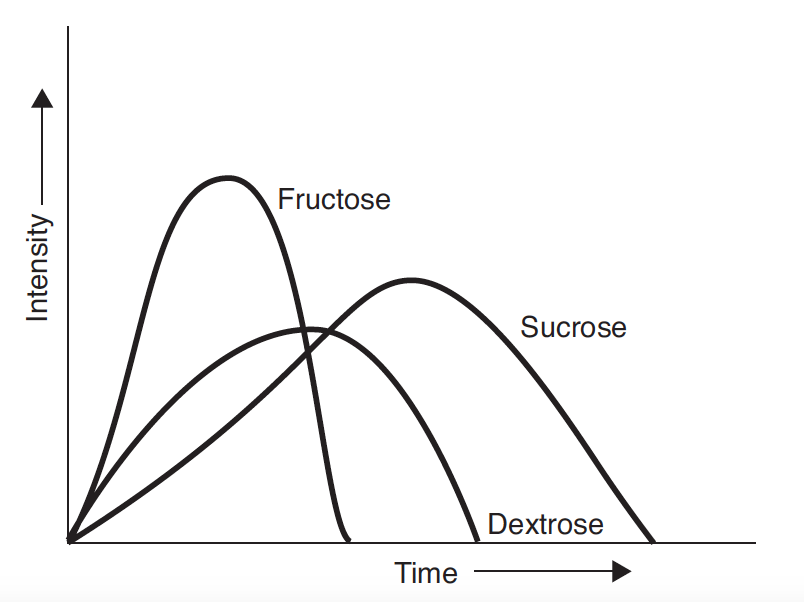
Pure crystalline dextrose (dextrose monohydrate) is the ultimate end product of starch hydrolysis. It is a moderately sweet sugar that is less sweet than either fructose or sucrose, and has a perceived sweetness that lasts for longer than that of fructose, but less than that of sucrose (Hull, 2010). Dextrose enhances flavour, has a low viscosity, and lowers the freezing point nearly twice as much as sucrose. This results in ice cream that is soft and easy to scoop, but melts quickly and has a reduced shelf-life during storage. One interesting property of dextrose is that it has a negative heat of solution; that is when it dissolves, there is a cooling effect, which makes it very compatible with mint flavours. Below is a profile of sweetness response, from Hull (2010).
6. Fructose
With further enzyme processing, dextrose can be converted to fructose, the sweetest of the natural sugars. In its pure form, fructose is a white crystalline material, although it is more frequently used as a component of high fructose corn syrups (HFCS). HFCS contain primarily fructose and dextrose with lesser quantities of higher sugars. The most commonly used is HFCS 42%. Fructose and HFCS are very sweet, enhance flavour, have low viscosity, and, like dextrose, lower the freezing point nearly twice as much as sucrose.
7. Why is corn syrup used in ice cream?
The use of corn syrup in ice cream is generally perceived to enhance flavour; provide enhanced smoothness, creaminess, and body; reduce recrystallisation during storage, which improves the shelf life of the ice cream; provide better meltdown characteristics; make the ice cream softer and easier to scoop; stabilise and reduce the size of air bubbles, and to provide an economical source of solids (Goff & Hartel, 2013).
7.1. Flavour enhancement
Corn syrups will often enhance or mask the perception of flavour. Generally, high DE syrups have a greater flavour enhancement than low DE syrups, which either mask or reduce the perception of flavour. Hull (2010) explains that as the taste receptors on our tongues are situated in depressions on the surface, low DE syrups are unable to enter these pores because the large molecules of the higher sugars are too large to reach the receptor sites. Conversely, high DE syrups, which contain fewer higher sugars, are able to enter the pores, and are therefore more able to carry the flavours to the taste receptors, making them ideal syrups for transferring flavours.
Fructose has a rapidly forming and intense sweet taste that is perceived earlier than that of sucrose or dextrose, and that dissipates more quickly than sucrose and dextrose. Because of this, fructose enhances flavour. Fruit flavours, particularly citrus, spices, and acid come through more clearly and distinctly after the fructose sweetness dissipates because they are not masked by the lingering sweetness of sucrose. This makes fructose an ideal sweetener for fruit sorbets.
7.2. Enhance smoothness and creaminess
As well as enhancing flavour, corn syrups enhance smoothness and creaminess through three main mechanisms: 1. specific effects on ice crystallisation; 2. effect on freezing point depression; and 3. effect on the viscosity of an ice cream mix.
7.2.1. Ice crystallisation
Smooth and creamy ice cream requires the majority of its ice crystals to be small. If many crystals are large, the ice cream will be perceived as being coarse or icy. To control ice crystal size, it is important to develop an understanding of ice formation (known as crystallisation) during the freezing of ice cream. Ice cream is frozen in two stages, the first being a dynamic process where the mix is frozen in a scraped-surface freezer (SSF) (an ice cream machine) whilst being agitated by the rotating dasher, a mixing device with sharp scraper blades attached, to incorporate air, destabilise the fat, and form ice crystals. Upon exiting the SSF, the ice cream, at about -5°C to -6°C (23°F to 21.2°F) and with a consistency similar to soft-serve ice cream, undergoes static freezing where it is hardened in a freezer without agitation until the core reaches a specified temperature, usually -18°C (-0.4°F).
During dynamic freezing, the ice cream mix is added to the SSF at between 0°C and 4°C (32°F and 39.2°F). As the refrigerant absorbs the heat in the mix, a layer of water freezes to the cold barrel wall causing rapid nucleation (the birth of small ice crystals) (Hartel, 2001). The crystals that form at the cold barrel wall are then scraped off by the rotating scraper blades and dispersed into the centre of the barrel where warmer mix temperatures cause some crystals to melt and others to grow and undergo recrystallisation.
Recrystallisation is defined as “any change in number, size, shape… of crystals” (Fennema, 1973) and basically involves small crystals disappearing, large crystals growing, and crystals fusing together, all of which result in an overall increase in ice crystal size. Russell et al. (1999) found that crystallisation during the freezing of ice cream is dominated by recrystallisation and growth and that these mechanisms appear to be more important than nucleation in determining the final crystal population.
In general, as the concentration of a sweetener is increased, ice crystals become smaller owing to a reduction in ice crystal growth rate and delayed nucleation during dynamic freezing (Omran & Kind, 1974; Hartel, 1996; Haddad Amamou et al., 2010). This effect can be explained by two facts. First, the higher viscosity promotes crystal melting and attrition. Second, the solution has a higher resistance to water diffusion (movement of melted liquid from smaller ice crystals to the surface of larger ice crystals) at higher concentrations of sweetener (Haddad Amamou et al., 2010).
7.2.2. Freezing point depression
The freezing point of pure water is 0°C (32°F). When a substance is dissolved in water, however, the temperature at which the water freezes is lowered. This lowering of the freezing point is referred to as the ‘Freezing Point Depression’ and is defined as the difference between 0°C (32°F) and the temperature at which water in an ice cream mix first begins to freeze (Goff & Hartel, 2013). Freezing point depression is influenced primarily by sweeteners (including the lactose in milk) and milk salts. Increasing the amount of these solutes will lower the freezing point of an ice cream mix, resulting in less ice being formed at a given temperature.
Different sweeteners depress the freezing point of water to different extents, depending on the number of small molecules in the mix. The lower the molecular weight of a sweetener, the greater the effect it will have on lowering the freezing point. Dextrose and fructose, having nearly half the molecular weight of sucrose, will be twice as effective at lowering the freezing point than an equivalent weight of sucrose. 20 DE CSS will actually cause an increase in the freezing point compared with that for sucrose.Freezing point depression affects the rate of recrystallisation during static freezing, the softness and scoopability of ice cream, and the rate at which ice cream melts during consumption.
7.2.2.1. Recrystallisation during storage
As ice cream sits in storage, the ice crystals continually grow by recrystallisation (Donhowe & Hartel, 1996; Hartel, 1998). This increase in crystal size eventually reaches a point where the ice cream develops coarse texture, at which point it has surpassed its shelf life. Several studies have found a direct relationship between recrystallisation rate and freezing point; that is, the lower the freezing point, the higher the recrystallisation rate during storage (Hagiwara & Hartel, 1996; Harper & Shoemaker, 1983; Miller-Liveney & Hartel, 1997). This is because as the freezing point is depressed, the amount of unfrozen water increases, and this unfrozen water will participate readily in recrystallisation during storage.Investigating the effects of various sweeteners (sucrose, 20 DE CSS, 42 DE CSS, and 42% HFCS) and stabilizers on ice recrystallisation during storage, Hagiwara & Hartel (1996) found that ice creams containing HFCS exhibited the highest recrystallisation rates, whereas ice creams made with 20 DE or 42 DE CSS had the lowest recrystallisation rates. These findings were attributed to the greater freezing point depression caused by HFCS (-4.4°C (24°F)) compared to 20 DE CSS (-1.7°C (28.9°F)).
7.2.2.2. Melting rate
The type and amount of sweetener also affects the melting rate of ice cream during consumption, with a lower freezing point leading to an increased rate of melting (Muse & Hartel, 2004; Junior & Lannes, 2011; Goff & Hartel, 2013). Ice cream made with either dextrose or fructose will have a higher melting rate because of a lower freezing point, whereas ice cream made with maltodextrin or 20 DE CSS will have a slower melting rate because of a higher freezing point.
7.2.2.3. Softness and scoopability
Sweeteners are also responsible for the softness and scoopability of ice cream through their effect on freezing point depression. Corn syrup products with a lower molecular weight will generally produce soft ice cream owing to a low freezing point and the subsequent reduction in the ice phase volume (the amount of frozen water). Ice cream made with maltodextrin or 20 DE CSS, which both have a high molecular weight, will likely have a high freezing point and hard texture. If sucrose is replaced with either dextrose or fructose, the freezing point is likely to be low, resulting in less frozen water and softer ice cream.
7.2.3. Viscosity
Smooth and creamy texture, primarily associated with a high milk fat content and small ice crystal size, is also strongly influenced by the viscosity of the ice cream mix (Mela et al., 1994; Akhtar et al., 2005). Viscosity can be loosely defined as the thickness of a liquid, with thicker liquids having higher viscosities (honey has a higher viscosity than water for example). In general, as the viscosity of an ice cream mix increases, the smoothness of texture, body, and resistance to melting increases, but the amount of air, or overrun, decreases (Marshall et al., 2003).
The viscosity of a mix increases with increasing concentration of stabiliser, protein, corn syrup, fat, and total solids, with the contribution of each decreasing in that order (i.e. stabiliser has more influence on mix viscosity than does fat) (Goff & Hartel, 2013). Maltodextrins and Low DE corn syrups are very viscous due to the higher sugars they contain. When added to an ice cream mix, they will add body and increase mix viscosity. Because high DE syrups contain very few higher sugars, they will have a minimal effect on increasing the viscosity of an ice cream mix.
Muse & Hartel (2004) used either sucrose, 42 DE HFCS, or 20 DE corn syrup as the sweetener to investigate the factors that influence the melting rate and hardness of ice cream. The researchers found that the mix containing 20 DE CS was the most viscous and that made with HFCS was the least viscous. Similarly, Ozdemir et al. (2003) found that the use of HFCS instead of sucrose decreased the viscosity and overrun (air content).
7.2.3.1. Stability and size of air bubbles
The viscosity of an ice cream mix also plays an important role in the stability and size of air bubbles. During dynamic freezing, the small, newly formed air bubbles are not stable and need to be stabilised to prevent coalescence. Coalescence involves the coming together of two or more bubbles and results in larger air bubble sizes (Ronteltap & Prins, 1989). It is important to minimise this coalescence since smaller dispersed air cells produce a creamier mouthfeel during consumption (Eisner et al., 2005). The addition of corn syrups enhances the stability and reduces the size of air bubbles through their effect on increasing the viscosity of the ice cream mix. This makes maltodextrins and low DE syrups, with their viscosity enhancing ability, ideal for ice cream production.
7.3. Total solids
Ice crystal size is related inversely to the total solids (the fat, milk solids-not-fat, sweetener, egg yolk solids, stabiliser, and emulsifier) of an ice cream mix; that is, ice cream made from a mix with a higher total solids content generally contains smaller ice crystals (Donhowe et al., 1991; Guinard et al., 1997). The theory behind this is that an increase in the level of total solids in the mix will lower the amount of water and thereby reduce the total amount of ice formed. Because of their low sweetness value, maltodextrins, corn syrup (20 to 64 DE), and maltose, are a convenient and cost-effective way of increasing total solids without introducing excessive sweetness.
8. Summary
Corn syrups are nutritive sweeteners produced by the partial hydrolysis of corn starch. As the DE of a syrup increases, so too does its sweetness, flavour enhancement, and effect on freezing point depression, but its viscosity, ability to enhance smoothness and creaminess, contribution of body, mouthfeel, and solids, air bubble stabilisation, and ability to increase the shelf-life of ice cream during storage decrease.Maltodextrins and low DE corn syrups have very little sweetness, produce a firmer and chewier ice cream that melts slowly, reduce the rate of recrystallisation during storage, thus increasing shelf life, and promote the formation of small ice crystals and air cells through their viscosity-enhancing properties and contribution to total solids. Excessive use of maltodextrins or low DE corn syrups, will, however, generally mask or reduce the perception of flavour. 28-42 DE corn syrups are generally the most commonly used corn syrup in ice cream production and are used in combination (3-5%) with sucrose (10-12%).Dextrose is the ultimate end product of starch hydrolysis. With further enzyme processing, dextrose can be converted to fructose, as in the production of high fructose corn syrups. Both dextrose and fructose syrups contribute sweetness, enhance flavour, especially fruit flavours, and produce a softer ice cream that is easier to scoop but melts faster. Excessive use of dextrose or fructose will likely result in excessive sweetness, a soft ice cream that melts too quickly, and an increased rate of recrystallisation during storage, which will limit the shelf life of the ice cream. I hope this review of corn syrup products helps. Do feel free to get in touch with any questions or suggestions on how this post can be improved. Ruben :)
7. References
Akhtar, M., Stenzel, J., Murray, B. S., and Dickinson, E., 2005. Factors affecting the perception of creaminess of oil-in-water emulsions. Food Hydrocolloids, 19. 521-526.
Donhowe, D. P., Hartel R. W., and Bradley R. L., 1991. Determination of ice crystal size distributions in frozen desserts. J. Dairy Sci. 74.
Donhowe, D. P., and Hartel, R. W., 1996. Recrystallization of ice in ice cream during controlled accelerated storage. Int Dairy J, 6 (11–12):1191–208.
Eisner, M. D., Wildmoser, H., and Windhab, E, J., 2005. Air cell microstructuring in a high viscous ice cream matrix. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 263(1)
Goff, H. D., and Hartel R. W., 2013. Ice Cream. Seventh Edition. New York: Springer.
Guinard, J. X., Zoumas Morse, C., Mori, L., Uatoni, B., Panyam, D., and Kilara, A., 1997. Sugar and Fat Effects on Sensory Properties of Ice Cream. Journal of Food Science. 62.5.
Haddad Amamou, A., Benkhelifa, H., Alvarez, G., and Flick, D., 2010. Study of crystal size evolution by focused-bean reflectance measurement during the freezing of sucrose/water solutions in a scraped-surface heat exchanger. Process Biochemistry. 45. 1821-1825.
Hagiwara, T., and Hartel, R. W. 1996. Effect of sweetener, stabilizer, and storage temperature on ice recrystallization in ice cream. J Dairy Sci. 79(5):735–44.
Harper, E. K., and Shoemaker, C. F., 1983. Effect of locust bean gum and selected sweetening agents on ice recrystallization rates. J. Food Sci. 48:1801.
Hartel, R. W., 1996. Ice crystallisation during the manufacture of ice cream. Trends in Food Science & Technology. 7(10).
Hartel, R. W., 1998. Phase transitions in ice cream. In: RaoMA, Hartel RW, editors. Phase/state transitions in foods: chemical, structural, and rheological changes. IFT basic symposium series. New York: Marcel Dekker. p 327–68.
Hartel, R. W., 2001. Crystallisation in foods. Gaithersburg, MD: Aspen Publishers.
Hull, P., 2010. Glucose Syrups, Technology and Applications. Singapore: Wiley-Blackwell.
Junior, E. D. S., and Lannes, S. C. D. S., 2011. Effect of different sweetener blends and fat types on ice cream properties. Cienc. Tecnol. Aliment., Campinas. 31(1), 217-220.Marshall, R. T., Goff, H. D., and Hartel R. W., 2003. Ice cream (6th ed). New York: Kluwer Academic/Plenum Publishers.
Mela, D. J., Langley, K. R., and Martin, A., 1994. Sensory assessment of fat content: effect of emulsion and subject characteristics. Appetite, 22, 67–81.
Miller-Livney T., and Hartel, R. W., 1997. Ice recrystallization in ice cream: interactions between sweeteners and stabilizers. Journal of Dairy Science. 80:447–56.
Muse, M. R., and Hartel, R. W., 2004. Ice Cream Structural Elements that Affect Melting Rate and Hardness. Journal of Dairy Science. 87, 1-10.
Omran, A. M., and King, C. J., 1974. Kinetics of ice crystallization in sugar solutions and fruit juices. AIChE Journal. 20(4). 795–803.
Ozdemir, C., Dagdemir, E., Ozdemir, S., and Sagdic, O., 2008. The effects of using alternative sweeteners to sucrose on ice cream quality. Journal of Food Quality. 32. 415-428.
Ronteltap, A. D., and Prins, A., 1989. Contribution of drainage, coalescnece, and disproportionation to the stability of aerated foodstuffs and the consequences for the bubble size distribution as measured by a newly developed optical glass-fibre technique. In: Bee, R. D., Richmond, P., and Mingings, J., (Eds), Food colloids. The proceedings of an international symposium organised by the food chemistry group of the royal society of chemistry. Coloworth, United Kingdom.
Russell, A. B., Cheney, P. E., and Wantling, S. D., 1999. Influence of freezing conditions on ice crystallisation in ice cream. Journal of Food Engineering. 29.